usp class vi elastomers
PlasticsToday Staff Sep 17 2015 The flexibility durability neutrality and recyclability of the MT-1205I TPE make it a desirable alternative to PVC and thermoset rubber for medical tubing according to Polymax. CFR 1772600 USP Class VI to 70C 11 Si 871 TR FDA 21.

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
PPE develops novel elastomer materials to meet the most demanding sealing applications including extreme temperatures and chemically aggressive environments.

. CFR 1772600 USP Class VI to 70C 3-A Sanitary Standard 12 VMQ Si 70 W FDA 21. Ia USP Class VI andor ISO 109933 will be required. Both products are optically clear and they meet the USP class VI specifications.
Food and Drug Administration issues regulations for material in contact with food. CFR 1772600 USP Class VI to 121C 3-A Sanitary Standard 9 10 12 Vi 971 W FDA 21. Most applications are fairly benign to elastomers.
The elastomers like neoprene are perfect for this. Provides a high-level introduction to elastomer chemistry manufacturing technology and the post processing of components 3. The Brooks Instrument mass flow controller model SLA Biotech can offer certification of compliance with US.
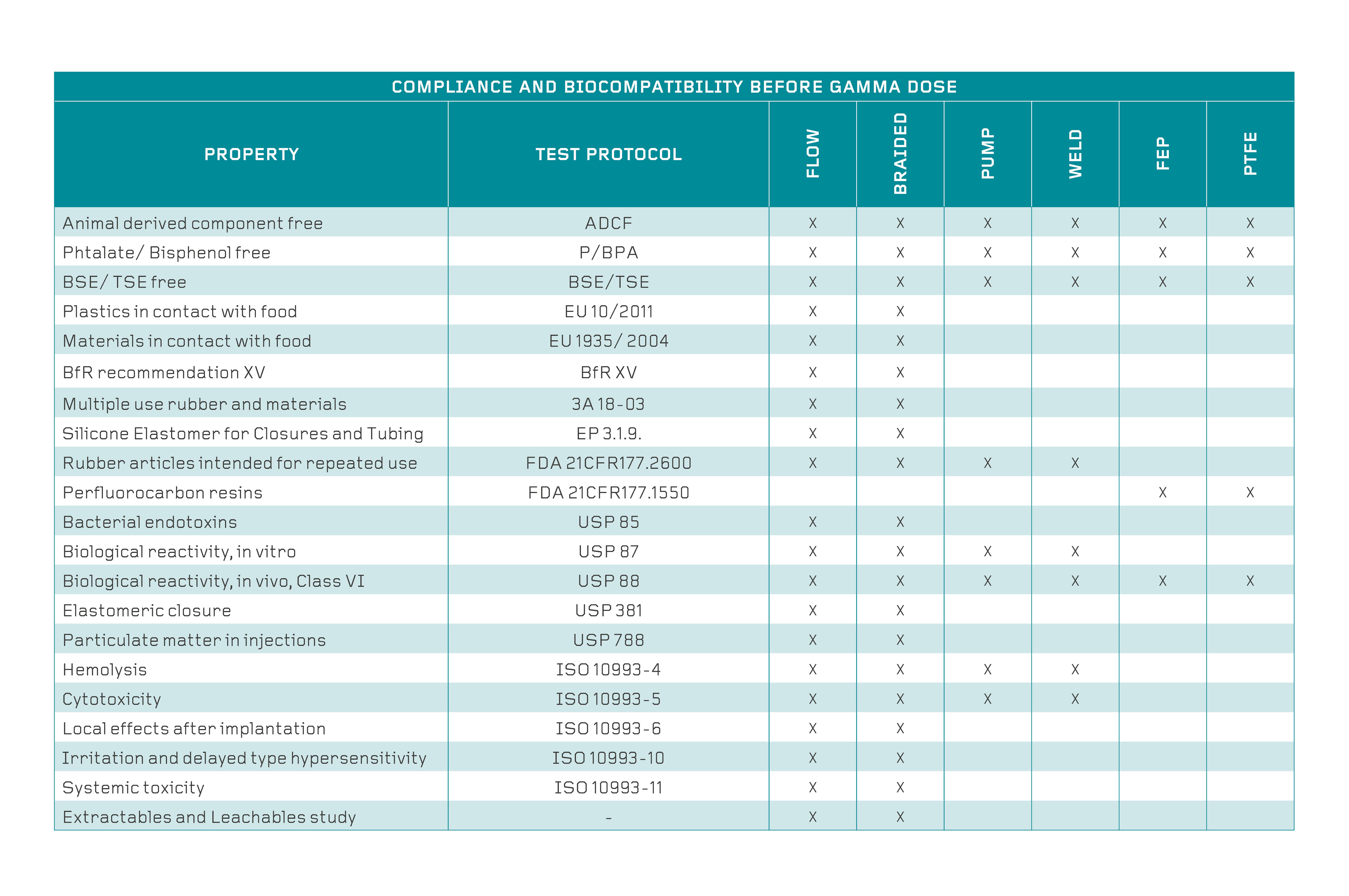
Elastomer like silicon has widely used the material to build them and many other goods. Ecdel 9967 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required.
Frequently Asked Questions FAQs. Pharmacopeia USPs Class VI standards for plastics. This item has been discontinued by the manufacturer and is no longer available.
For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. Medical field needs a wide range of products like prosthetics lubricants molds with superior class of chemical and thermal resistance. Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance for flexible packaging including medical and pharmaceutical applications.
Based on the environment different elastomers can be recommended. Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by gamma irradiation and autoclave Product Validation Test Summaries available upon request Moldable bondable and formable for single-use assemblies and overmolds Temperature. They also have models available that certify that no animal derivatives were used in the raw materials of the elastomer.
ISO 9001 FDA USP Class VI. Making certain assumptions including the assumption that the bonding area is completely accessible to UV light we can recommend UV10Med or UV10TKMed. Ecdel 9966 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance needed in a variety of flexible packaging including flexible medical and pharmaceutical packaging applications.
Master Bond offers a variety of USP Class VI approved UV curable compounds. FEPM Vi 602 FDA 21. Please call customer service for assistance.
Ecdel 9967 may be processed on standard injection molding extrusion blow. However some applica-tions such as implantable devices are extremely complicated. Explains basic functional characteristics of components 4.
MIL-STD-883J for thermal stability. Inner Dimension - 0375. Please contact the division for assistance in selecting materials in these situations.
The Premium Package includes all elastomers in the wetted path of the SLA Series Biotech including O-rings and Valve Seats are USP Class VI and ADI Free and include a full package of certificates. Table 1 shows our standard programme FDA compliant com-pounds which can be produced in a few days. CFR 1772600 USP Class VI to 121C 9 12 FKM Vi 780 FDA 21.
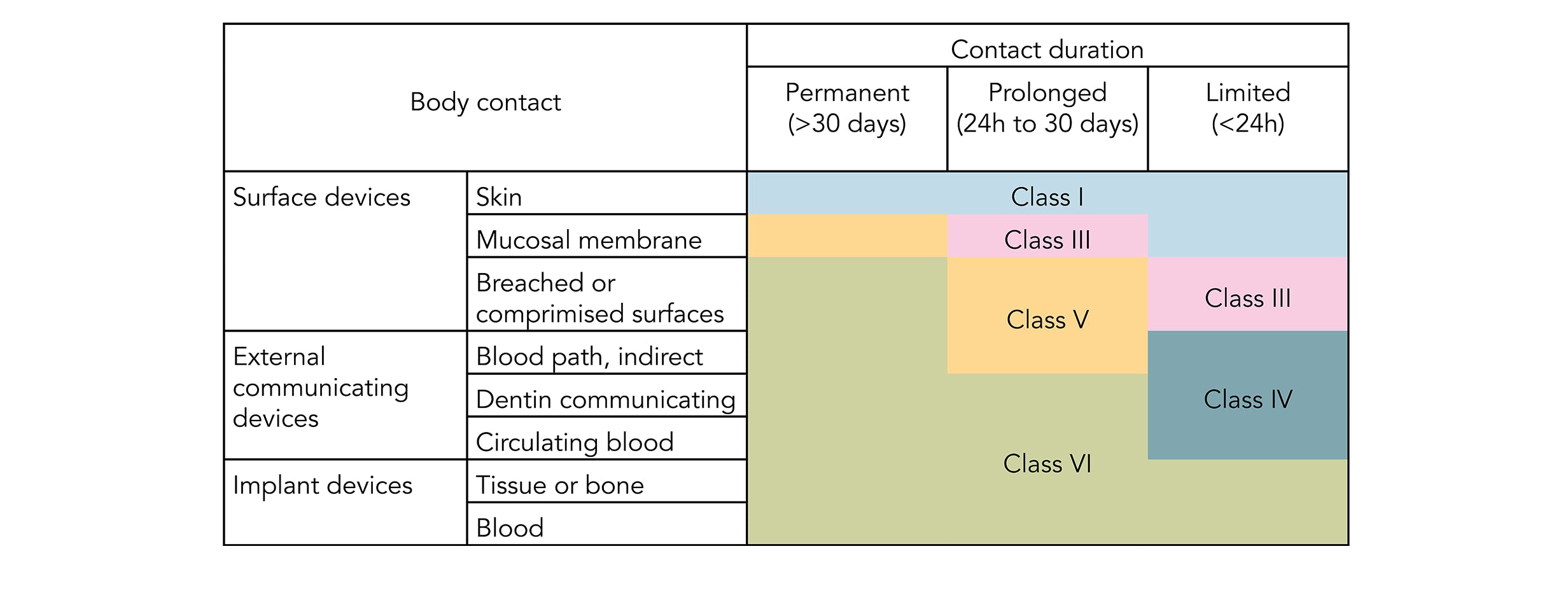
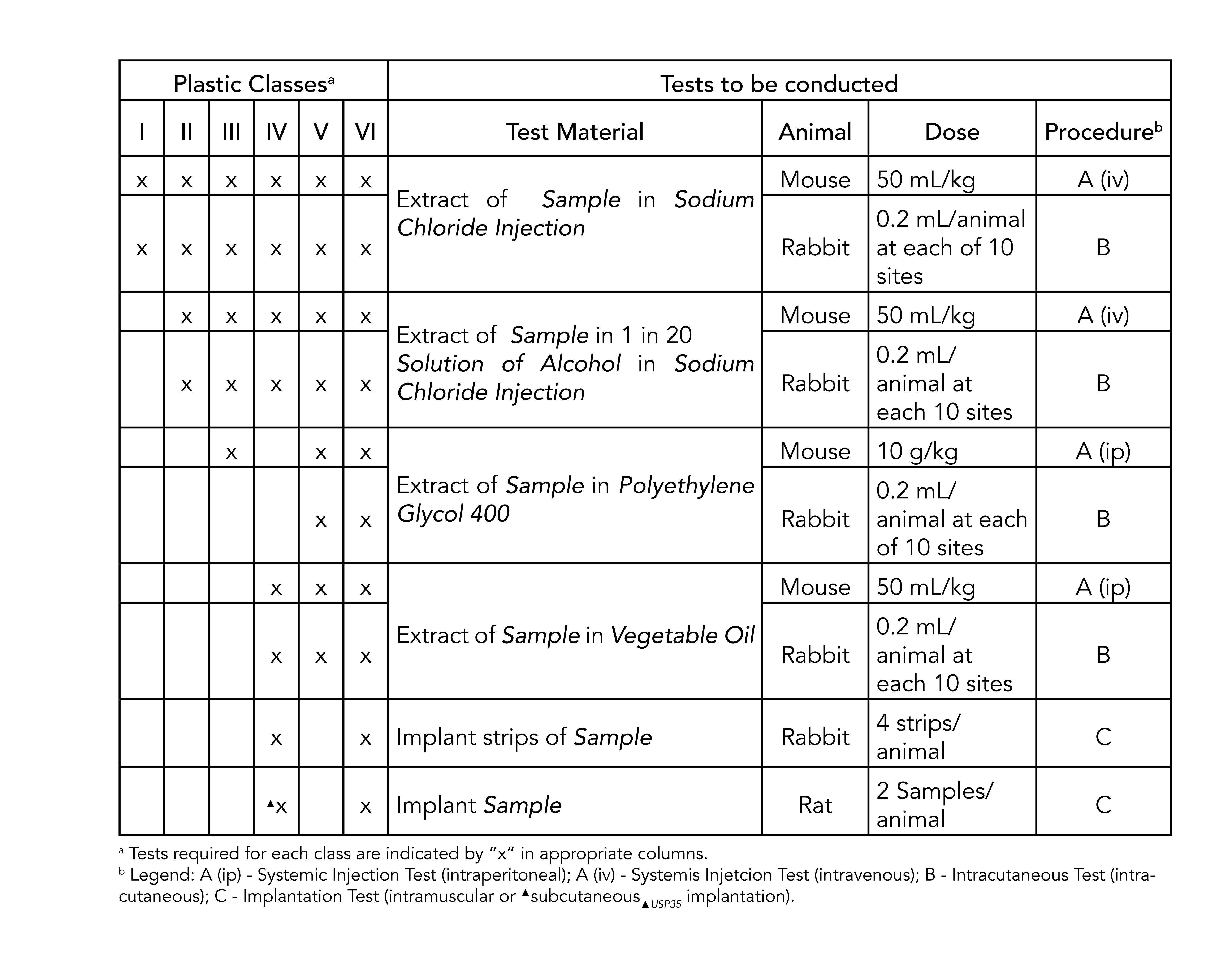
Most applications are fairly benign to elastomers. Plastics were assigned Class I-VI based on the biological in vivo testing systemic injection intra-cutaneous and implantation tests. Sil 714002 USP class VI Silicone 1 70 Yes transl.
Discusses identification testing A workshop Modernization of USP Packaging Standards for Glass and. FAR 25853a for flame retardancy. In addition UV10TKMed also meets the ISO 10993-5.
The transparent elastomer is a high-purity compound with low leachables and extractables as required by the USP Class VI standard for ensuring patient safety. Additionally 22 Material Certifications certifying the composition of all materials in the wetted path and International Calibration Traceability calibrations traceable to NIST or. UL 1203 for explosion-proof.
Tubing USP Class VI Thermoplastic Elastomer meets requirementsfor plastic containers and closures Female MPC and Polycarbonate meets USP Class VI requirementsfor plastics. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.
USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts. View substitute products 11-189-14. Expert seal design advice can be provided and components manufactured in the shortest lead times in the sealing industry.
UL FDA NSF USP Class VI ASTM JIS SAE MIL Specs RoHS Reach. MIL-STD-810G for fungus resistance. In 1988 in vitro tests were explored and USP concluded that in vitro.
Stay Compliant with USP Class VI for your mass flow controller. UL 94V-0. What is TPE.
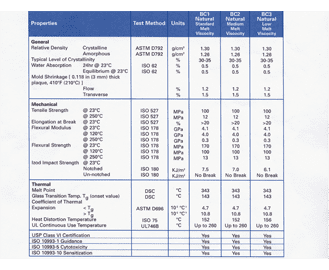
Sil 714001 USP class VI Silicone 1 70 Yes transl. Silicone S0317-60 A red colored material that offers the widest temperature range of all the material choices - going from -65F to 400F. This material is good in dry heat and has good ozone resistance.
UV10TKMed is USP Class VI approved and meets ISO10993-5 for cytotoxicity requirements. ISO 10993-5 for cytotoxicity. The USP Class VI approved elastomers are.
The central regulation for elastomer materials is contained in 21 CFR 1772600 - Rubber articles intended for repeated use. FDA CFR 175300. Designates baseline requirements 5.
Class VI materials testing includes system toxicity and intracutaneous toxicity. USP Class VI for biocompatibility. It is used in the assembly of medical devices and is capable of withstanding repeated sterilizations including radiation ethylene oxide chemical sterilants and especially autoclaving.
7 USP Class VI materials. 1965 USP XVII introduced Biological TestsPlastics Containers section was added and made official in the Compendium.

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Silicones Wacker Silicones For Medical Why Silicones Extremely

Parker Medical Fda O Rings Sealing Devices

Meaning Of Usp Class Vi Standard United Kingdom

Fda And Usp Class Vi O Rings Guide 2020 Nes

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
![]()
Usp Vi Silicones Fda Approved Pronat

Usp Class Vi Seals Precision Polymer Engineering

Usp Class Vi Seals Compliant To Food Grade Standards Barnwell

Pharmaceutical And Cosmetics Production